Context
The identification of unmet health-related needs is crucial for the development of a needs-based healthcare policy and innovation. Meeting unmet needs is a common objective of many stakeholders in the health system. Although there is no single definition of unmet health-related needs, there is a consensus that these can be approached both from the perspective of the individual patient and from the societal perspective.
In the NEED project, health-related needs are defined as the specific needs related to a particular health condition, which encompasses the needs of individuals affected by the condition and possible broader societal needs that result from the impact of the condition on segments of society not directly afflicted by the condition (e.g. transmissibility, impact on informal caregivers, environmental impact).
Identifying health-related needs must happen in a scientifically valid manner to allow for evidence-based decision making along the lifecycle of health-related interventions, from development to reimbursement, post-implementation follow-up and application. There is currently no research structure in place to develop, organise and/or coordinate this scientific activity, while the expertise, experience and part of the data are available at several Belgian federal institutions. In 2021, KCE advised the minister of public health to mandate an existing independent scientific organisation to take up the responsibility of coordinating unmet needs research, generating a solid evidence base and exploring possible international collaborations for this activity (see link).
Figure: Foundations for a needs-driven health care system: from assessment to appraisal and use
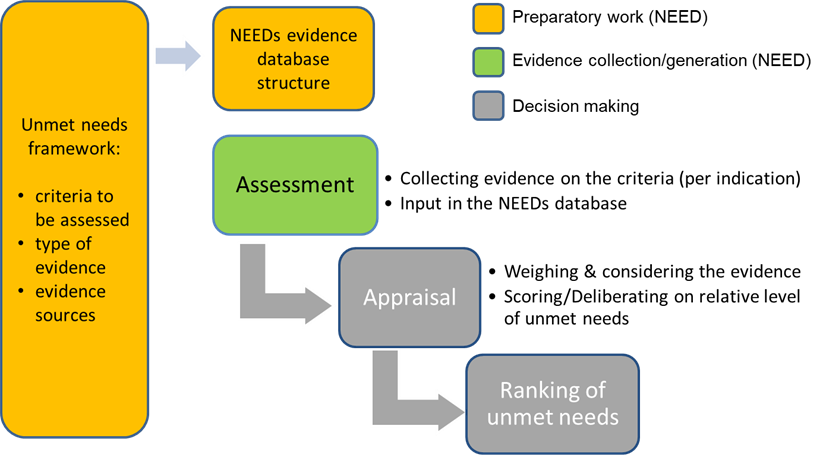
Objectives
The overall aim of NEED (Needs Examination, Evaluation and Dissemination) is to develop an independent research infrastructure that collects evidence on unmet health-related needs, both from the patients’ perspective and that of society, and stores it in a database that can be used by all relevant stakeholders. The availability of explorable evidence on patient and societal needs is a prerequisite for moving from a supply-driven system towards a more needs-driven system. NEED should provide the infrastructure and content to allow for such policies.
- Objective 1: Define the NEED database structure, including the criteria to assess patient and societal needs, the type of evidence that has to be collected for each criterion, the possible evidence sources and their related quality.
- Objective 2: Start filling the NEED database, by collecting evidence on patient and societal needs related to health issues with potential high unmet needs.
- Objective 3: Disseminate the information of NEED to all relevant stakeholders, both nationally and internationally, ensuring data protection (especially for rare diseases) and secure secondary use of data.
Impact
The role of NEED can best be described as “providing the evidence base required to make an evidence-based appraisal of the level of (patient or societal) needs related to different health conditions”.
NEED’s place in the healthcare sector is overarching, as it serves multiple stakeholders, including researchers, research funders, regulators, reimbursement decision makers, patient associations, and public services responsible for public health, industry or healthcare providers. Stakeholders at national and international level will benefit from the evidence base provided by the NEED database.
NEED will lead to a number of relevant innovations for Belgian and European policy-making and society:
- Belgium Presidency of the Council of the EU (S1 2024): NEED will inform the Council conclusions Belgium will formulate as part of its work within the “Health and Healthcare” pillar, by providing a proof-of-concept for a proposed structure to identify patient and societal needs.
- Reform of the EU pharmaceutical legislation (April 2023, European Commission): although "unmet medical needs" is a central concept in the new legislation, a clear operational definition or procedure for identifying unmet medical needs is still lacking. NEED might provide input into the discussion at EU level on how to implement and use the concept of "unmet medical needs" in practice.
- European Medicines Agency (EMA): several of EMA’s Regulatory Science Research Needs to 2025 include research on (the identification of) unmet medical needs.
- EU Health Technology Assessment (HTA) network: knowledge on the needs of patients and society is crucial for joint clinical assessments of new pharmaceutical products and medical devices.
- Research activities: the European Commission’s DG Research & Innovation recently commissioned a group to study “high-burden under-researched conditions” in Europe and beyond with the objective to steer future research funding.

